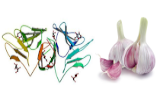
Allium sativum Lectin (ASA)
Common or cultivated garlic (Allium sativum) is a species of monocotyledonous perennial vegetable plant whose bulbs, with a strong smell and taste, are often used as a condiment in cooking.
Allium sativum lectin (ASA) is isolated from the garlic bulbs and purified by affinity chromatography followed by gel filtration. It is a dimer of two subunits with a molecular weight of 24,000 with an isoelectric point between pH 7.2 and pH 7.4.
The sugar specificities of this lectin can be elucidated by hemagglutination inhibition and a coupled enzyme assay. The hemagglutination inhibition study confirms the specificity of this lectin for D-mannose, while a sensitive enzyme assay allows detailed binding specificities to be examined. The ASA binding sites contain a number of mannose residues, with a sugar eluted from the mannose. Interaction with glycoproteins suggests that lectin not only recognizes internal mannose, but also binds to the central pentasaccharide of nitrogen-bound glycans, even when sialylated. Although the binding site may be resistant to terminal sialic acids and residues of the penultimate galactose, the removal of sialic acids increases potency.
Allium sativum lectin agglutinates rabbit erythrocytes but not human erythrocytes.
Search result : 52 product found
Refine your search :
RUOCE / IVD
- Unconjugated 16
- Agarose 2
- Biotin 2
- TRITC 2
- AP 1
- Cy3 1
- Cy5 1
- DyLight 488 1
- FITC 1
- HRP 1
- Texas Red® 1
- plant 16
- Lectins
Cat#
Description
Cond.
Price Bef. VAT
‹
›

