EnzyChrom™ NAD/NADH Assay Kit
Application
- For sensitive determination of NAD and NADH and evaluation of drug effects on NAD/NADH metabolism.
Key Features
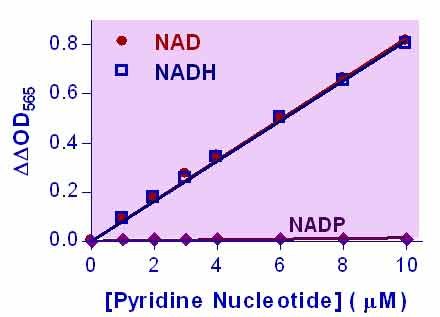
- Sensitive and accurate. The detection limit of 0.05 µM and linearity up to 10 µM NAD+/NADH in 96-well plate assay.
- Convenient. The procedure involves adding a single working reagent, and reading the optical density at time zero and 15 min at room temperature.
- High-throughput. Can be readily automated as a high-throughput 96-well plate assay for thousands of samples per day.
Method
- OD565nm
Samples
- Cell or tissue extracts
Species
- All
Procedure
- 15 min
Size
- 100 tests
Detection Limit
- 0.05 µM
Shelf Life
- 6 months
More Details
Pyridine nucleotides play an important role in metabolism and, thus, there is continual interest in monitoring their concentration levels. Quantitative determination of NAD+/NADH has applications in research pertaining to energy transformation and the redox state of cells or tissue. Simple, direct, and automation-ready procedures for measuring NAD+/NADH concentration are very desirable. BioAssay Systems EnzyChrom™ NAD+/NADH assay kit is based on a lactate dehydrogenase cycling reaction, in which the formed NADH reduces a formazan (MTT) reagent. The intensity of the reduced product color, measured at 565 nm, is proportional to the NAD+/NADH concentration in the sample. This assay is highly specific for NAD+/NADH and with minimal interference (<1%) by NADP+/NADPH. Our assay is a convenient method to measure NAD, NADH, and their ratio.When tissue is used, should it be freshly obtained? Or is -80°C storage ok?
If direct sample processing is not possible, we recommend snap freezing tissue samples in liquid nitrogen and keeping them either in liquid nitrogen or at -80°C until further processing.
Maeda, T., et al (2021). Relevance of nadh dehydrogenase and alternative two-enzyme systems for growth of corynebacterium glutamicum with glucose, lactate, and acetate. Frontiers in Bioengineering and Biotechnology, 8. Assay: NAD/NADH in C. glutamicum cells.
Paul, S., et al (2021). D4F prophylaxis enables redox and energy homeostasis while preventing inflammation during hypoxia exposure. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 133, 111083. Assay: NAD/NADH in male Sprague Dawley rats lung tissue.
Woodford, C., et al (2020). Nicotinamide promotes differentiation of pancreatic endocrine progenitors from human pluripotent stem cells through poly (ADP-ribose) polymerase inhibition. BioRxiv, 2020.04.21.052951. Assay: NAD/NADH in mouse cells.
Nonaka, D., et al (2021). Metabolic engineering of 1,2-propanediol production from cellobiose using beta-glucosidase-expressing E. coli. Bioresource Technology, 329, 124858. Assay: NAD/NADH in E. coli cells.
Di Magno, L., et al (2020). Phenformin inhibits hedgehog-dependent tumor growth through a complex i-independent redox/corepressor module. Cell Reports, 30(6), 1735-1752.e7. Assay: NAD/NADH in Math1-CRE;Ptch1 tissue.
Giroud-Gerbetant, J., et al (2019). A reduced form of nicotinamide riboside defines a new path for NAD+ biosynthesis and acts as an orally bioavailable NAD+ precursor. Molecular Metabolism, 30, 192-202. Assay: NAD/NADH in Mice tissue and cells.
Wang, X., et al (2019). Subcellular NAMPT-mediated NAD+ salvage pathways and their roles in bioenergetics and neuronal protection after ischemic injury. Journal of Neurochemistry, 151(6), 732-748. Assay: NAD/NADH in C57BL/6J mice neurons.
Li, R., et al (2020, August 7). Emodin alleviates hydrogen peroxide-induced inflammation and oxidative stress via mitochondrial dysfunction by inhibiting the pi3k/mtor/gsk3β pathway in neuroblastoma sh-sy5y cells [Research Article]. BioMed Research International. Assay: NAD/NADH in SH-SY5Y cells.
Xu, K., et al (2020, December 30). Vgll4 protects against oxidized-ldl-induced endothelial cell dysfunction and inflammation by activating hippo-yap/tead1 signaling pathway [Research Article]. Mediators of Inflammation. Assay: NAD/NADH in Ox-LDL-induced human umbilical vein endothelial cells.
Sato, N., et al (2020). Metabolic engineering of shikimic acid-producing corynebacterium glutamicum from glucose and cellobiose retaining its phosphotransferase system function and pyruvate kinase activities. Frontiers in Bioengineering and Biotechnology, 8. Assay: NAD/NADH in C. glutamicum cells cells.
Chen, X., et al (2020). Synergistic effect and mechanism of plumbagin with gentamicin against carbapenem-resistant klebsiella pneumoniae. Infection and Drug Resistance, 13, 2751-2759. Assay: NAD/NADH in Klebsiella pneumoniae cells.
Liu, W., et al (2019). Emodin inhibits zinc-induced neurotoxicity in neuroblastoma SH-SY5Y cells. Bioscience Reports, 39(BSR20182378). Assay: NAD/NADH in Human neuroblastoma SH-SY5Y cells.
Li, M., et al (2019). Effects of nadh availability on 3-phenyllactic acid production by lactobacillus plantarum expressing formate dehydrogenase. Current Microbiology, 76(6), 706-712. Assay: NAD/NADH in Lactobacillus plantarum cells.
Wada, K., et al (2020). Application of a pyruvate-producing escherichia coli strain lafcpcpt-accbc-acee: A case study for d-lactate production. Fermentation, 6(3), 70. Assay: NAD/NADH in E. coli HIT-DH5α cells.
Takaso, Y., et al (2020). Deletion of CD38 and supplementation of NAD + attenuate axon degeneration in a mouse facial nerve axotomy model. Scientific Reports, 10(1), 17795. Assay: NAD/NADH in mouse cells.
Byun, J., et al (2019). Both gain and loss of Nampt function promote pressure overload-induced heart failure. American Journal of Physiology-Heart and Circulatory Physiology, 317(4), H711-H725. Assay: NAD/NADH in mouse cells.
Sato, N., et al (2020a). Metabolic engineering of shikimic acid-producing corynebacterium glutamicum from glucose and cellobiose retaining its phosphotransferase system function and pyruvate kinase activities. Frontiers in Bioengineering and Biotechnology, 8. Assay: NAD/NADH in C. glutamicum cells.
Wen, Q., et al (2019). Characterization of balofloxacin-stressed proteomics and identification of balofloxacin-binding proteins pre-peptidase and integration host factor in Edwardsiella tarda. Journal of Proteomics, 205, 103413. Assay: NAD/NADH in E. tarda cells.
Fragola, G., et al (2020). Deletion of Topoisomerase 1 in excitatory neurons causes genomic instability and early onset neurodegeneration. Nature Communications, 11(1), 1962. Assay: NAD/NADH in Mouse cortices.
Deng, W., et al (2020). L-lysine potentiates aminoglycosides against Acinetobacter baumannii via regulation of proton motive force and antibiotics uptake. Emerging Microbes & Infections, 9(1), 639-650. Assay: NAD/NADH in A. baumannii cells.
Michon, C., et al (2020). A bacterial cell factory converting glucose into scyllo -inositol, a therapeutic agent for Alzheimer?s disease. Communications Biology, 3(1), 1-7. Assay: NAD/NADH in B. subtilis cells.
Liu, Y., et al (2020). Metabolic regulation of endothelial SK channels and human coronary microvascular function. International Journal of Cardiology, 312, 1-9. Assay: NAD/NADH in Human myocardial cells.
Forte, M., et al (2020). Pharmacological restoration of autophagy reduces hypertension-related stroke occurrence. Autophagy, 16(8), 1468-1481. Assay: NAD/NADH in Human A10 cells.
Bueno, M. J., et al (2019). Essentiality of fatty acid synthase in the 2D to anchorage-independent growth transition in transforming cells. Nature Communications, 10(1), 5011. Assay: NAD/NADH in nude mice cells.
Stoyas, C. A., et al (2020). Nicotinamide pathway-dependent sirt1 activation restores calcium homeostasis to achieve neuroprotection in spinocerebellar ataxia type 7. Neuron, 105(4), 630-644.e9. Assay: NAD/NADH in Mouse cerebellar granule neurons.
Walker, M. A., et al (2021a). Acetylation of muscle creatine kinase negatively impacts high-energy phosphotransfer in heart failure. JCI Insight, 6(3). Assay: NAD/NADH in Mouse cardiac tissue.
Xiao, Y., et al (2020). Inhibition of glucose assimilation in Auxenochlorella protothecoides by light. Biotechnology for Biofuels, 13(1), 146. Assay: NAD/NADH in Auxenochlorella protothecoides cells.
Maric, T., et al (2019). Bioluminescent-based imaging and quantification of glucose uptake in vivo. Nature Methods, 16(6), 526-532. Assay: NAD/NADH in 4T1-luc cells.
Kilfoil, P. J., et al (2019). Metabolic regulation of Kv channels and cardiac repolarization by Kvβ2 subunits. Journal of Molecular and Cellular Cardiology, 137, 93-106. Assay: NAD/NADH in mice heart tissue.
Lundt, S., et al (2020). The effect of NAMPT deletion in projection neurons on the function and structure of neuromuscular junction (Nmj) in mice. Scientific Reports, 10(1), 99. Assay: NAD/NADH in mice muscle tissue.
Wang, P., et al (2020). Increasing ascomycin yield in streptomyces hygroscopicus var. Ascomyceticus by using polyhydroxybutyrate as an intracellular carbon supply station. Assay: NAD/NADH in S. hygroscopicus var. ascomyceticus FS35 hyphae.
Khattab, S. M. R., & Watanabe, T. (2021a). Metabolic engineering of Saccharomyces cerevisiae for efficient conversions of glycerol to ethanol. BioRxiv, 2021.01.04.425180. Assay: NAD/NADH in E. coli cells.
Altamimi, T. R. (2018). Integrated Regulation of Cardiac Fatty Acid and Glucose Oxidation. Assay: NAD/NADH in mice heart tissue.
Paul, S., Gangwar, A., Bhargava, K., & Ahmad, Y. (2018). STAT3-RXR-Nrf2 activates systemic redox and energy homeostasis upon steep decline in pO2 gradient. Redox biology, 14, 423-438. Assay: NAD/NADH in sprague dewley rats tissue.
Wang, W., Hu, Y., Wang, X., Wang, Q., & Deng, H. (2018). ROS-Mediated 15-Hydroxyprostaglandin Dehydrogenase Degradation via Cysteine Oxidation Promotes NAD+-Mediated Epithelial-Mesenchymal Transition. Cell chemical biology, 25(3), 255-261. Assay: NAD/NADH in mice tissue.
Wang, Z., Jiang, M., Guo, X., Liu, Z., & He, X. (2018). Reconstruction of metabolic module with improved promoter strength increases the productivity of 2-phenylethanol in Saccharomyces cerevisiae. Microbial cell factories, 17(1), 60. Assay: NAD/NADH in yeast cells.
He, X., Wu, C., Cui, Y., Zhu, H., Gao, Z., Li, B. & Zhao, B. (2017). The aldehyde group of gossypol induces mitochondrial apoptosis via ROS-SIRT1-p53-PUMA pathway in male germline stem cell. Oncotarget, 8(59), 100128. Assay: NAD/NADH in cotton cells.
Qiao, A., Jin, X., Pang, J., Moskophidis, D., & Mivechi, N. F. (2017). The transcriptional regulator of the chaperone response HSF1 controls hepatic bioenergetics and protein homeostasis. J Cell Biol, 216(3), 723-741. Assay: NAD/NADH in mice liver tissue.
Bae, S. J., Kim, S., & Hahn, J. S. (2016). Efficient production of acetoin in Saccharomyces cerevisiae by disruption of 2, 3-butanediol dehydrogenase and expression of NADH oxidase. Scientific reports, 6, 27667. Assay: NAD/NADH in yeast cells.
Bai P et al (2011). PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 13(4):461-8. Assay: NAD/NADH in mouse cells.
Koo BS et al (2010). Improvement of coenzyme Q(10) production by increasing the NADH/NAD(+) ratio in Agrobacterium tumefaciens. Biosci Biotechnol Biochem.74(4):895-8. Assay: NAD/NADH in yeast Agrobacterium tumefaciens.
Lee M et al (2010). Depletion of GSH in glial cells induces neurotoxicity: relevance to aging and degenerative neurological diseases. FASEB J. 24(7):2533-45. Assay: NAD/NADH in human cell.
Hsu CP et al (2009). Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 105(5):481-91. Assay: NAD/NADH in mouse heart cardiac myocytes.
Tseng HC et al (2009). Metabolic engineering of Escherichia coli for enhanced production of (R)- and (S)-3-hydroxybutyrate. Appl Environ Microbiol. 75(10):3137-45. Assay: NAD/NADH in bacteria E.coli.
Clem B,et al (2008). Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther. 7(1):110-20. Assay: NAD/NADH in human epithelial cell.
Greenall A et al (2008). A genome wide analysis of the response to uncapped telomeres in budding yeast reveals a novel role for the NAD+ biosynthetic gene BNA2 in chromosome end protection. Genome Biol. 9(10):R146. Assay: NAD/NADH in yeast cell.
Kim Y, et al (2008). Dihydrolipoamide dehydrogenase mutation alters the NADH sensitivity of pyruvate dehydrogenase complex of Escherichia coli K-12. J Bacteriol. 190(11):3851-8. Assay: NAD/NADH in bacterial E. coli.
Olesen UH, et al (2008). Anticancer agent CHS-828 inhibits cellular synthesis of NAD. Biochem Biophys Res Commun. 367(4):799-804. Assay: NAD/NADH in human cell.
Song HK, et al (2008). Visfatin: a new player in mesangial cell physiology and diabetic nephropathy. Am J Physiol Renal Physiol. 295(5):F1485-94. Assay: NAD/NADH in human mesangial cells.
Thornburg JM et al (2008). Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. 10(5):R84. Assay: NAD/NADH in human breast adenocacinoma cell.
To find more recent publications, please click here.
If you or your labs do not have the equipment or scientists necessary to run this assay, BioAssay Systems can perform the service for you.
– Fast turnaround
– Quality data
– Low cost