Staurosporine [62996-74-1]
Referencia A8192-10mg
embalaje : 10mg
Marca : APExBIO Technology
Staurosporine
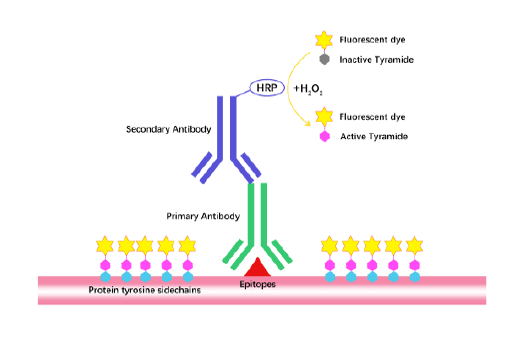
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
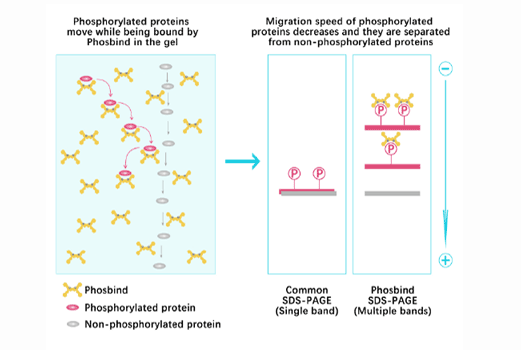
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
Staurosporine, an alkaloid produced in Streptomyces staurospores originally as an antifungal agent, is an inhibitor of a broad spectrum of protein kinases, including protein kinase C (PKC), Camp-dependent protein kinase (PKA), phosphorylase kinase, ribosomal protein S6 kinase, epidermal growth factor receptor (EGF-R) kinase and Ca2+/calmodulin-dependent protein kinase II (Ca/CaM PKII). The inhibition potency is strongest for PKC (IC50 = 2.7 nM) but several-fold lower for other protein kinases. Staurosporine exhibits a strong cytotoxicity to some mammalian tumor cell lines, induces cell apoptosis, and arrests fission yeast cell elongation specifically at a stage immediately after cell division.
Reference
Takashi Toda, Mizuki Shimanuki, and Mitsuhiro Yanagida. Fission yeast genes that confer resistance to staurosporine encode an AP-1-like transcription factor and a protein kinase related to the mammalian ERK1/MAP2 and budding yeast FUS3 and KSS1 kinases. Genes Dev. 1991 5: 60-73
Michelle M. Hill, Mirjana Andelkovic, Derek P. Brazil, Stefano Ferrari, Doriano Fabbro, and Brian A. Hemmings. Insulin-stimulated protein kinase B phosphorylation on Ser-473 is independent of its activity and occurs through a Staurosporine-insensitive kinase. J. Biol. Chem 2001, 276: 25643-25646.
Flavio Meggio, Arianna Donella Deana, Maria Ruzzene, Anna M. Brunati, Luca Cesaro, Barbara Guerra, Thomas Meyer, Helmut Mett, Doriano Fabbro, Pascal Furet, Grazyna Dobrowolska, and Lorenzo A. Pinna. Different susceptibility of protein kinases to staurosporine inhibition kinetic studies and molecular bases for the resistance of protein kinase CK2. Eur. J. Biochem. 234, 317-322 (1995)
- 1. Arwen Conod, Marianna Silvano, et al. "On the origin of metastases: Induction of pro-metastatic states after impending cell death via ER stress, reprogramming, and a cytokine storm." Cell Rep. 2022 Mar 8;38(10):110490. PMID: 35263600
- 2. Ramon Edwin Caballero, Simon Xin Min Dong, et al. "Role of RIPK1 in SMAC mimetics-induced apoptosis in primary human HIV-infected macrophages." Sci Rep. 2021 Nov 25;11(1):22901. PMID: 34824340
- 3. Zintis Inde, Jason Rodencal, et al. "Quantification of drug-induced fractional killing using high-throughput microscopy." STAR Protoc. 2021 Jan 22;2(1):100300. PMID: 33532743
- 4. Chu xue, Si-Xue Liu, et al. "Corydalis Saxicola Bunting Total Alkaloids Inhibits Paclitaxel-Induced Peripheral Neuropathy By Regulating PKCε-TRPV1 and p38 MAPK-TRPV1 Signaling Pathways." Research Square. doi.org/10.21203/rs.3.rs-332970/v1.
- 5. Zintis Inde, Giovanni C. Forcina, et al. "Kinetic Heterogeneity of Cancer Cell Fractional Killing." Cell Rep. 2020 Jul 7;32(1):107845. PMID: 32640215
- 6. Zintis Inde, Giovanni C. Forcina, et al. "Large-Scale Analysis of Cell Death Phenotypic Heterogeneity." bioRxiv 2020.02.28.970079.
- 7. Waer CN, Kaur P, et al. "Rosmarinic Acid/ Blue Light Combination Treatment Inhibits Head and Neck Squamous Cell Carcinoma In Vitro." Anticancer Res. 2020;40(2):751–758. PMID: 32014917
- 8. Mikhail Chesnokov, Imran Khan, et al. "The MEK1/2 pathway as a therapeutic target in high-grade serous ovarian carcinoma." bioRxiv. 2019 September 16.
- 9. Clark R, Usselmann L, et al. "A flexible high content imaging assay for profiling macrophage efferocytosis." J Immunol Methods. 2019 Jul 29:112636. PMID: 31369739
- 10. Chung HK, Zou X, et al. "A compact synthetic pathway rewires cancer signaling to therapeutic effector release. Science." 2019 May 3;364(6439). PMID: 31048459
- 11. Ashok Kumar, Ramon Edwin Caballero, et al. "Inhibitor of apoptosis, IAP, genes play a critical role in the survival of HIV-infected macrophages." BioRxiv. 2019 February 06.
- 12. Wang Y, Li Y, et al. "The cerebral cavernous malformation disease causing gene KRIT1 participates in intestinal epithelial barrier maintenance and regulation." FASEB J. 2018 Sep 25:fj201800343R. PMID: 30252535
- 13. Nissen SK, Pedersen JG, et al. "Multiple Homozygous Variants in the STING-Encoding TMEM173 Gene in HIV Long-Term Nonprogressors." J Immunol. 2018 May 15;200(10):3372-3382. PMID: 29632140
- 14. Jarrett Smith,Geraldine Seydoux, et al. "Liquid-like P granules require ATP hydrolysis to avoid solidification." bioRxiv,Jan. 10, 2018.
- 15. Melanie Villanueva, B.Sc. "Glyoxalase 1 Attenuates the Effects of Chronic Hyperglycemia on Explant-Derived Cardiac Stem Cells." University of Ottawa.Melanie Villanueva, B.Sc.
- 16. Bozza, William P., et al. "The Use of a Stably Expressed FRET Biosensor for Determining the Potency of Cancer Drugs." PloS one 9.9 (2014): e107010. PMID: 25188024
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 466.53 |
| Cas No. | 62996-74-1 |
| Formula | C28H26N4O3 |
| Solubility | insoluble in H2O; insoluble in EtOH; ≥11.66 mg/mL in DMSO |
| Chemical Name | (5S,6R,7R,9R)-6-methoxy-5-methyl-7-(methylamino)-6,7,8,9,15,16-hexahydro-17-oxa-4b,9a,15-triaza-5,9-methanodibenzo[b,h]cyclonona[jkl]cyclopenta[e]-as-indacen-14(5H)-one |
| SDF | Download SDF |
| Canonical SMILES | O=C(NC1)C2=C1C3=C(C4=C2C5=C(C=CC=C5)N4[C@H]6C[C@@H](NC)[C@@H](OC)[C@]7(C)O6)N7C8=CC=CC=C83 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment: | |
| Cell lines | A31 cell lines, CHO-KDR cell lines, Mo-7e cell lines and A431 cell lines. |
| Preparation method | The solubility of this compound in DMSO is |
| Reaction Conditions | 24 h; IC50=0.08 mM (A31 cell lines), IC50=0.30 mM (Mo-7e cell lines), IC50=1.0 mM (CHO-KDR cell lines). |
| Applications | Staurosporine inhibited the ligand-induced autophosphorylation of the receptors for platelet-derived growth factor (PDGF) (IC50=0.08 mM) in A31 cell lines, stem cell factor (c-Kit, IC50=0.30 mM) in Mo-7e cell lines, and for VEGF (KDR, IC50=1.0 mM) in CHO-KDR cell lines, but did not affect the ligand-induced autophosphorylation of the receptors for insulin, IGF-I, or epidermal growth factor (EGF) in A431 cell lines. |
| Animal experiment: | |
| Animal models | Athymic nude mice |
| Dosage form | 75 mg/kg/day; oral taken. |
| Applications | The treatment with Staurosporine (75 mg/kg/day p.o.) completely inhibits the angiogenic response to VEGF, but not to bFGF. Thus, Staurosporine may suppress tumor growth by inhibiting tumor angiogenesis (via its effects on the VEGF-R tyrosine kinases) in addition to directly inhibiting tumor cell proliferation (via its effects on PKCs). This anti-angiogenic action may contribute to the antimetastatic and broad antitumor activity displayed by Staurosporine, as well as the synergy with cytotoxic agents. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Andrejauskas-Buchdunger E, Regenass U. Differential inhibition of the epidermal growth factor-, platelet-derived growth factor-, and protein kinase C-mediated signal transduction pathways by the staurosporine derivative CGP 41251[J]. Cancer research, 1992, 52(19): 5353-5358. [2] Fabbro D, Buchdunger E, Wood J, et al. Inhibitors of protein kinases: CGP 41251, a protein kinase inhibitor with potential as an anticancer agent[J]. Pharmacology & therapeutics, 1999, 82(2): 293-301. | |
| Description | Staurosporine is a potent inhibitor of PKC for PKCα, PKCγ and PKCη with IC50 values of 2 nM, 5 nM and 4 nM, respectively. | |||||
| Targets | PKCα | PKCγ | PKCη | PKCδ | PKCε | PKCζ |
| IC50 | 2 nM | 5 nM | 4 nM | 20 nM | 73 nM | 1086 nM |
Related Biological Data

Related Biological Data

Related Biological Data

Related Biological Data

Related Biological Data

Related Biological Data